The Effects of Lead on Biological Models: Enzyme Function, Cell Membrane Permeability, and Cell Regeneration
Abstract
Years of mining for heavy metals have resulted in neglected mineshafts and towering chat piles that are major sources of water contamination all over the United States. The Tar Creek drainage basin in Northeastern Oklahoma and Southeastern Kansas is one of the many areas of the world with high lead levels affecting the health of people around it. Picher, a ghost town in Northeastern Oklahoma, was once a large site for mining of lead and zinc ores from about 1900 until 1970. Recent findings of the EPA indicate that no level of lead intake is safe. Research indicates that lead toxicity leads to severe medical problems including brain damage, Alzheimer’s, heart disease, and damage to the bodies’ ability to detoxify. This project focuses on the effects of the heavy metal lead on biological assays. The purpose of this investigation is to determine the effects of low levels of lead on three biological systems. The three systems tested will be enzyme function, cell membrane permeability, and cell regeneration. Catalase will be the enzyme tested, beets will be used to represent the cell membrane, and Lumbriculus variegatus will be used to study cell regeneration. It is hypothesized that exposure to low levels of lead will inhibit enzyme function, inhibit cell growth, and will inhibit cell membrane functions. Materials used for this investigation includes lead nitrate diluted to 20 ppb, 10 ppb, 5 ppb, 2.5 ppb and 1.25 ppb. Lead dilutions were used to test for the ability of catalase, a common detoxifying enzyme, to catalyze the decomposition of hydrogen peroxide to water and oxygen. Lead dilutions were used to determine the effects on cell membrane permeability using beets. As the permeability of the cell membrane is compromised by lead, betacyanin, a red beet pigment diffuses into the solution. Absorbance was measured with a colorimeter. Finally, lead dilutions were used to determine the effect on cell regeneration. Lumbriculus variegatus, a common mud worm used in ecotoxicity testing, were cut into three sections, placed in lead concentrations and observed for cell regeneration. According to the data collected, low levels of lead significantly inhibited enzyme function, inhibited cell membrane function by increasing the permeability of the membrane to plant pigments and affected the survival rate and segment regeneration in Lumbriculus variegatus.
Review of the Literature
Years of mining for heavy metals have resulted in neglected mine shafts and towering chat piles that are major sources of water contamination all over the United States. The Tar Creek drainage basin in Northeastern Oklahoma and Southeastern Kansas is one of the many areas of the country with high lead levels affecting the lives of residents who live around it. Picher, a ghost town in Northeastern Oklahoma, was once a large site for mining of lead and zinc ores from about 1900 until 1970. There are approximately 1,300 mineshafts in the Oklahoma portion of the Picher Mining District, many of which can still be accessed. (16). Due to storm water runoff, many lakes and rivers surrounding these mines have become contaminated with various heavy metals causing health problems to those who ingest the water (8). Unfortunately, water was not the only thing contaminated. Chat from the old mines was distributed as sand and gravel fill for playgrounds, schoolyards, ball fields, and roadways (13). The Tar Creek site was added to NPL in 1983 (18).
Lead is a naturally occurring heavy metal with toxic effects to organisms and their environments at very low levels. Lead can be found within the dust in homes, or maybe could be found in the parks or playgrounds young children play near, and might even be in tap water. The EPA has decided that there is no safe level of lead intake which is very disturbing when you think about the forty-two states in America that exceed the legal limits- 15 parts per billion of lead in drinking water while other states do not perform the required testing. Chlorine is the most common element found in tap water with lead coming in as a close second (14). According to Dr. Peter Montague of the Environmental Research Foundation, studies have proven that lead in water is possibly the largest source of lead in the human blood stream. The effects of lead are irreversible and cumulative (13). Lead can be passed from mother to fetus during gestation or through breast-feeding causing the infant to suffer from severe medical conditions all through out his or her life (6). In adults, lead contaminated water can cause high-blood pressure and can reduce hemoglobin production, necessary for oxygen transport, and can interfere with normal cellular calcium metabolism (13). Lead is absorbed just like calcium and is incorporated into the bone, where it is stored. The stored lead is released when the body is under physical stress or if certain minerals become sparse (13). Lead causes the messages sent to every cell and organ by the brain to become distorted and unclear. Lead uptake can be reduced in the presence of high levels of iron and calcium (1)(11).
Lead is a larger problem for children than it is for adults. Severe brain damage and mental retardation can be seen in young children as a result of high lead levels. Other affects of lead on children include reduced IQs, depression, anxiety, behavioral problems such as hyperactivity and reduced attention spans (15). Additionally, scientists feel that the toxin lead may have an affect on adolescent crimes and anti-social behaviors in children (15). The heartbreaking fact about lead contamination in drinking water is that it is completely preventable. By taking a few simple steps, the appalling affects of lead can be eliminated from homes and kept away from children. This is incredibly important according to Dr. Herbert Needleman, a psychiatrist at the University of Pittsburgh Medical Center, who stated, "Lead is a brain poison that interferes with the ability to restrain impulses" (15). According to Thomas O’ Halloran PhD, a research scientist at Northwestern University, "It is surprising how little is known about the cell biology of the effects of the transitional metals. Living systems respond to heavy metal stress at several levels, depending on whether the stress involves metal starvation or overload (16). Studies indicate that heavy metals inhibit plant growth because they denature proteins, such as enzymes, that control cellular processes (14). Studies indicate that heavy metals such as cadmium, mercury and lead inhibit drug-metabolizing enzymes of the lung and liver (7). Catalase enzyme is commonly found in living tissue, especially the liver. Catalase is a detoxifying enzyme that converts toxic peroxides to harmless products of water and oxygen. Enzymes are catalytic proteins that speed up metabolic reactions by lowering energy barriers and are essential to life. Enzymes work quickly, typically taking part in about a thousand reactions per second. The region on the enzyme where the substrate (reactant to be changed) attaches is called the enzyme’s active site. At that site, the substrate is changed slightly by the enzyme so that a specific chemical bond is weakened. This weakened chemical bond allows the substrate to break apart quickly. Some enzymes cause compounds to break apart while others cause compounds to form. Enzymes provide a way to speed up life’s essential functions without raising the temperature of an organism’s body (3). Heavy metal damage to enzymes causes a serious threat to the function of the cell and will be investigated in this project.
Another vital structure for the survival of a cell is the cell membrane. Very little research has been conducted on what lead does to the cell membrane of eukaryotes. But research about lead and bacteria indicates that heavy metals have a lethal effect on bacteria. The effectiveness of heavy metals on bacteria is due to the attraction of cell membrane proteins to metal ions. The effect is significant even when though the concentration of metal ions in solution may be as small as a few parts per million. The cells die because of the accumulative affects of heavy metal ions within the cell (2). The cell membrane can be described as the protective barrier for a cell that regulates what enters and leaves the cell. It is composed of a lipid bilayer that has both a polar and non-polar portion. The cell membrane is often described as a fluid mosaic of phospholipids and proteins. The lipid bilayer is arranged in a manner so that the hydrophilic, -OH group is toward the surface creating a polar region on both the exterior and interior surfaces. The middle, so called lipid "tails", are non-polar in structure. The polar region is susceptible to damage by lead (12). Beet tissue can be used to represent the living condition of the biological membrane. As the beets are subjected to various lead concentrations, stress will be placed on the biological membrane. If the beet membranes are damaged, betacyanin (a red pigment) will leak into the surrounding liquid. The intensity of color in the fluid should be proportional to the amount of damage sustained by the membrane. A colorimeter can be used to analyze the color intensity. As the lead damages the biological membrane, the beet pigment will leak into the solution and will color the solution a shade of pink-red. A higher concentration of colored solution absorbs more light and transmits less light then a solution of lower concentration. The colorimeter monitors the amount light that passes through the solution and is received by the photocell as an absorbance value. The greater the absorbance value; the greater the damage to the biological membrane (20).
In a quest for further answers, cell growth under various levels of lead will be researched by using Lumbriculus variegatus. Phil Ross, a research scientist from the Colorado School of Mines, studied the toxicity and bioaccumulation of metals on Lumbriculus variegatus in sediments of mine drainage. Ross’s research focused on the use of Lumbriuculus variegatus for exotoxicity studies for determining the presence of heavy metals in sediments (18). Lumbriculus variegatus; also know as the California black worm, is a freshwater annelid that can be found just beneath the water’s surface at the edges of muddy ponds and marshes. Black worms are usually about one to two inches long and consist of anywhere from 150 to 250 segments that can be observed through transparent skin (10). California mud worms can be cut into many pieces without bleeding or dying and can regenerate a new head or tail, or both, from severed pieces. Morphallaxis, or self-fragmentation, in Lumbriculus variegatus usually takes twenty-one days (two to three weeks) and is the process by which the worms reproduce asexually (4). While several references can be found concerning the use of Lumbriculus to study cell regeneration, this research project focuses on connecting cell regeneration in Lumbriculus to the presence of heavy metals.
The purpose of this investigation is to study the effects of low levels of Lead on three biological systems. The three systems tested will be enzyme function, cell membrane permeability, and cell regeneration. Catalase will be the enzyme tested, beets will be used to represent the cell membrane, and Lumbriculus variegatus will be used to study cell regeneration.
It is hypothesized that exposure to low levels of lead will inhibit:
The null hypothesis states that there will be no differences between the experimental groups exposed to lead solutions and the Control group exposed to spring water only.
Materials
Lumbriculus variegatus Wards Biological Supply 85W4681
Catalase Enzyme from Bovine Liver Wards Biological Supply 38W2370
Beet Tissue Local Grocer
Motic® 2000 Microscope Camera
0.1 M Lead nitrate Beakers 2% Potassium permanganate
Dilution Chamber 1.0 M Sulfuric acid 13 Well Plates
1.5% Hydrogen peroxide Stopwatch Vernier CBL®
Distilled Water Flasks Forceps
TI-83® Plus Calculator Vernier® Colorimeter Micropipette 1-10 uL
Swift® Microscope Ruler mm Reaction Surface
Spring Water Graduated Cylinder Single Edge Razor Blades
Procedures
Preparing Dilutions of Lead nitrate
1. 6 uL of a 0.1 M Lead nitrate solution was added to 1000 mL of spring water (this makes the 100% solution 20 ppb). Lead nitrate was selected for this test because it is a very soluble form of lead in water.
2. Using a dilution chamber, dilute the 20 ppb concentration to 10, 5, 2.5 and 1.25 ppb.
3. Keep the dilutions as a stock solution for each bioassay.
Establishing a Baseline data for Catalase Enzyme Test (See Appendix A for the reaction series for this test)
1. Mix 0.111 g of the enzyme Catalase into 1000 ml of spring water and keep on ice
2. Dilute Hydrogen peroxide to a 1.5% solution (store-bought is 3%)
3. Put 10 mls of Hydrogen peroxide into a small beaker
4. Add 3 mls of spring water to the Hydrogen peroxide.
5. Allow the catalase reaction to occur for 3 minutes. Stir occasionally.
6. To stop the reaction after 3 minutes, add 10 mls of 1.0 M sulfuric acid.
7. Remove a 5 mL sample into another clean beaker, and assay for the amount of Hydrogen peroxide remaining as follows. Using a 10 mL syringe to add Potassium permanganate a drop at a time to the solution until the solution turns purple or brown. Record how much Potassium permanganate was used to react all of the uncatalyzed Hydrogen peroxide. By figuring out how much peroxide was remaining, you can determine how effective the enzyme was.
Establishing the effects of Lead Dilutions on Catalase
1. Repeat the above procedures with the exception of adding 1.0 mL of the Lead nitrate dilution(s) (20 ppb, 10 ppb, 5 ppb, 2.5 ppb, and 1.25 ppb) to the enzyme solution for 3 minutes prior to testing.
2. Repeat the assay for uncatalyzed catalase for each concentration of lead for 3 trials of data.
Cell Membrane Permeability Test (Procedures for Setting up the CBL and Colorimeter are located in Appendix B)
1. Put 3 mls of each lead concentration (20 ppb-1.25 ppb) into four wells of a well plate (spring water for control)
2. Cut one fresh beet into twenty-four 0.5 cm3 pieces. Place the freshly cut beet cubes onto a moist paper towel to insure that the beet tissue will not dry out before the experimentation.
3. After all the cubes are cut, blot a moist paper towel on the beet tissue to remove all excess pigments and to secure your data.
4. Place one beet cube in each filled well and stir every 1-2 minutes for ten minutes. After the ten minutes, remove the tissue from the wells and observe the red-pink color of the solutions. The darker colored solutions indicate that the concentration tested severely damaged the beet’s cell membrane.
lead solutions. Record results.
Cell Regeneration Test-Lumbriculus variegatus
1. Prepare well plates by putting 1 ml of the Lead nitrate dilutions in each well. Set up 2 well-plates for each dilution series and the control.
2. Pipette the Lumbriculus variegatus into the top of a reaction surface. Cut each worm twice; once where the head and middle sections connect; and once where the middle and tail section connect. These sections are visible under the dissecting microscope by changes in the color pattern of the worm.
3. Distribute worms into the well plates using 12 worms for each solution.
4. Put a 1cm X 1cm fragment of paper towel into each well to allow the worms a surface to attach and a food source.
5. Check worms daily for mortality and function. After 10 days pipette each surviving worm on a slide and cover with a glass cover slip. Place the slide on 10 X to watch and count the newly regenerated segments. The new segments will be lighter in color and appear "spiky" when compared to the original segments.
Results
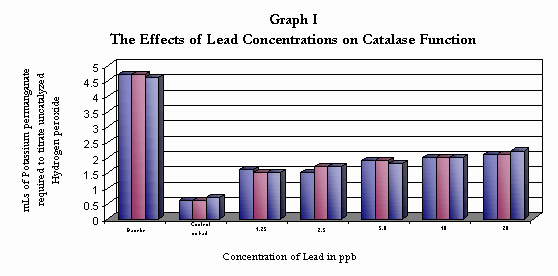
Data Table I
The Effects of Exposure to Various Lead Concentrations on Catalase Function
measured by the mls of Potassium permanganate required to react the hydrogen peroxide remaining in solution after 3 minutes enzyme reaction time
|
Baseline |
Control |
1.25 ppb | 2.5 ppb | 5.0 ppb | 10.0 ppb | 20 ppb | |
| Trail I Trail II Trail III Trail IV | 4.7 4.7 4.6 |
0.6 0.6 0.7 |
1.6 1.5 1.5 |
1.5 1.7 1.7 |
1.9 1.9 1.8 |
2.0 2.0 2.0 |
2.1 2.1 2.2 |
| Average |
4.66 |
0.63 |
1.53 |
1.63 |
1.86 |
2.0 |
2.1 |
Data Table II
The Effects of Exposure to Various Lead Concentrations on Cell Membrane Function
as measured by the presence of betacyanin in solution (absorbance)
|
Control |
1.25 ppb | 2.5 ppb | 5.0 ppb | 10.0 ppb | 20 ppb | |
| Trail I Trail II Trail III Trail IV |
0.16 0.19 0.18 0.18 |
0.19 0.20 0.19 0.18 |
0.21 0.19 0.21 0.20 |
0.20 0.24 0.21 0.22 |
0.24 0.21 0.23 0.22 |
0.31 0.27 0.21 0.25 |
| Average |
0.17 |
0.19 |
0.20 |
0.22 |
0.23 |
0.26 |
Data Table III
Total Number of Segments Regenerated in Lumbriculus variegatus
Exposed to Various Concentrations of Lead
| Control |
1.25 ppb | 2.5 ppb | 5 ppb | 10 ppb |
20 ppb | |
| 64 87 75 103 82 90 84 75 69 82 |
8 22 40 12 31 32 |
39 43 41 24 28 8 |
19 6 18 6 |
10 12 2 7 17 16 |
7 8 11 3 | |
| Average 81 | Average 24 |
Average 29 |
Average 12 | Average 10.6 | Average 7.25 | |
Data Table IV
The Percentage of Survival Rate in Lumbriculus variegatus
| Control |
1.25 ppb | 2.5 ppb | 5.0 ppb | 10 ppb | 20 ppb |
| 100% |
60% |
60% |
40% |
50% |
30% |
The baseline indicates the amount of Hydrogen peroxide in a 10 mL sample of a 1.5 % Solution
The Control indicates the Catalase enzyme function with out the presence of a lead, showing that the enzyme catalyzed most of the hydrogen peroxide within three minutes and left in solution only a small amount of hydrogen peroxide. The Lead exposed Catalase showed a reduced ability to catalyze the hydrogen peroxide with the 20 ppb sample leaving almost half of the hydrogen peroxide unreacted
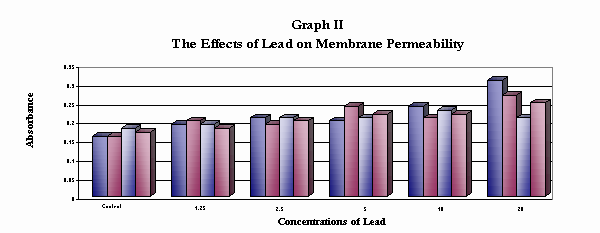
This graph shows the effect of low levels of lead on the cell membrane. Lead ions damage the cell membrane and pigment
concentration increases in the solution. As the lead concentration increases, the pigments increase in concentration
and absorbance increases.

This graph shows the regeneration of body segments exposed to lead concentrations. As lead concentrations increased regeneration of total segments decreased.

This graph indicates the survival rate of Lumbriculus variegatus regenerating in lead solutions. As the lead concentration increases survival rate decreased.
Discussion
The results of this investigation support research conducted by Ryoji Nakazawa, PhD and Yoshio Inouye, PhD concerning the effects of heavy metals on enzyme functions. Research suggests that heavy metals act to denature enzymes and inhibit their functions. Concentrations of lead ranging from 1.25 ppb to 20 ppb significantly inhibited catalase activity. This would suggest that lead levels similar to blood levels that would be considered alarming are capable of significant damage to cellular functions involving enzymes.
Although little literature research was found to indicate the effects of lead on eukaryotic cell membranes, data suggests that the lead levels tested increase cell membrane permeability and could provide valuable information into the way in which lead begins to attack cellular structure. This would support the research conducted with bacteria.
Research conducted by Drewes and Ross, indicate that Lumbriculus variegatus is a good model for exotoxicity tests of lead sediments in aquatic systems. Lumbriculus variegatus have been used to determine the presence of heavy metals in sediments commonly linked to superfund sites. The results of this investigation support the findings that low level exposure to lead is toxic to organisms as measured by cellular regeneration.
Conclusions
The hypothesis of this project stated that exposure to low levels of lead would inhibit enzyme function. According to the data collected for lead levels of 1.25 ppb to 20 ppb, enzyme function was significantly inhibited by the presence of low levels of lead when compared to the control. ANOVA analysis of the data gives strong evidence to reject the null hypothesis and accept the hypothesis (p=0.0001). It was hypothesized that exposure to low levels of lead would inhibit cell membrane functions by increasing the permeability of the membrane to plant pigments. According to the data collected, exposure to lead increased the permeability of the cell membrane compared to the control. As the concentration of lead increased, the amount of pigment in the solution increased and the absorption increased. This would indicate that lead ions have the ability to affect the stability of the cell membrane. The ANOVA indicated that the differences between the groups tested and the control were not due to chance giving strong evidence to reject the null hypothesis and accept the hypothesis (p=0.008).
It was also hypothesized that exposure to low levels of lead would inhibit cell growth as modeled by cell regeneration in Lumbriculus variegatus. Cell regeneration data indicates that lead significantly reduced cell regeneration. The control regenerated an average of 81 total body segments; while the regeneration in 1.25 ppb solutions regenerated an average of 24.2 total body segments, the 2.5 ppb regenerated an average of 16.3 total body segments, the 5.0 ppb solution regenerated an average of 12.25 total body segments, the 10 ppb solution regenerated an average of 10.6 total body segments, the 20 ppb solution only regenerated an average of 7.25 total body segments. There was a significant decrease in the amount of cell regeneration in cells exposed to low level lead concentrations. ANOVA analysis indicated strong evidence to reject the null hypothesis and accept the hypothesis (p= 0.014).
While the control group showed a 100% survival rate and good segment regeneration, the experimental groups were greatly affected by the exposure to lead. With only a 52% survival rate overall in the solutions tested (60% in 1.25 ppb; 60% in 2.5 ppb; 40% in 5 ppb; 60% in 10 ppb and only 40% in 20 ppb).
A future study indicated could be to determine how the lead affects the neurological behavior of a bioassay model. Or, it would be interesting to see if the worms exposed to lead would show a different regeneration rate after a recovery time and to see how long that recovery time would be.
Literature
Cited
2 Benson, Harold. Microbiological Applications. 7th Edition. McGraw-Hill ©1998 pg 115.
3 Campbell, Neal; Jane Reece; and Lawrence Mitchell. "Selections on Enzymes" Biology Fifth Edition. Addison Wesley. ©2001 page 124-136.
4 Drewes, Charles, PhD. "Those Wonderful Worms" Carolina Tips [Available online] http://www.carolina.com/tips 12/13/02.
5 Ground-Water Contamination by Heavy Metals: Tar Creek, Oklahoma http://toxics.usgs.gov/sites/heavy_metals.html 1/16/03
6 "How to protect your child from lead poisoning" [Available online]7 Inouye, Yoshio, et al. "Comparative Study on in vitro Inhibitory Effects of Heavy Metals on Rabbit Drug-Metabolizing Enzymes" Journal of Health Sciences, 47, 14-20, 2001.
8 "Lead" [Available online] http://www.epa.gov/cgi-bin/epaprintonly.cgi
9 "Lead Toxicity" [Available online] (8).
10 "Lumbriculus variegates" " [Available online] http://yale.edu/ynhti/curriculum/units/1997/7/97.07.05.x.html
11 "Medical effects of lead poisoning" [Available online] http://leadpoisonfacts.com/medicaleffcts.asp
12 Miller, Kenneth, Ph.D.; and Joseph Levine Ph.D. " Cell membranes" Biology Prentice Hall © 2002
13 Montague, Peter. "Health Effects of Lead" " [Available online] http://www.webhart.net/lead/leadhealth. 1/1/03
14 Nakazawa, R, et al. "Interaction between cadmium and nickel". Man and Environment. 26, 124-129, 2000.
15 Needleman, Herbert. "Lead-poisoning: What You Should Know" [Available online] http://healthynewage.com/lead.
16 O’ Halloran, Thomas. "Heavy Metals in Concert with Life", The Journal of Biological Chemistry. Vol 274, 15041-15045. 2002. [Available online]
17 "Quality of water in abandoned lead/zinc mines in Ottawa county, Oklahoma" [Available online] http://ok.water.usgs.gov/proj/picher.mine.html 1/16/03
18 Ross, Philip "Role of Dietary Exposure for Bioaccumulation and Toxicity of Metals in Aquatic Ecosystems affected by Mining" [Available Online] www.mines.edu/academic/envsci
19 "Tar Creek" [Available Online] www.health.state.ok.us/program/envhlth/Ottawa 12/20/02
20 Vernier, David. Biology with the CBL Vernier Software, Portland, OR © 2000 16-24.
Acknowledgements
Appendix
Appendix A
Reactions for the assay for catalase function are as follows:
2 H2O2 + Catalase à 2 H2O + O2
2 H2O2 + Catalase + Lead à 2 H2O + O2 + uncatalyzed H2O2
Uncatalyzed 5 H2O2 + 2 KMnO4 + 3 H2SO4 à K2SO4 + 2 MnSO4 + 8 H2O + 5 O2
KMnO4 will react with the H2O2 and be colorless
When all the H2O2 is reacted, the excess KmnO4 will cause the solution to be pink or brown
Appendix B
Procedures for Setting up the CBL and Colorimeter are located in Appendix B
1. Connect the Colorimeter to the CBL in channel one. Select the Applications button.
2. On the Applications menu select DataMate. Select Colorimeter prepare to calibrate the probe.
3. Fill a cuvette with distilled water to act as a blank to calibrate the colorimeter. Be sure to wipe the sides of the cuvette with Kimwipes and check the solutions for bubbles that might interfere with readings.